Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Takayama, Yusuke; Kikuchi, Hirohito*
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 27(1), p.12 - 21, 2020/06
In this study, an applicability of the modified Cam clay model to the buffer material under saltwater conditions was examined. First, consolidated-undrained triaxial test was conducted using NaCl solution and artificial seawater. Based on the consolidated-undrained triaxial compression test results and the existing consolidation test results, the difference in the mechanical behavior of the buffer material under distilled water and saltwater condition was clarified. In particular, there was a difference in the unloading behavior in the consolidation test. Through reproducibility analysis of these experimental data, it was confirmed that the mechanical behavior of the buffer material can be roughly reproduced by setting the swelling index according to the salt concentration.
*; Mihara, Morihiro;
JNC TN8430 2001-007, 56 Pages, 2002/01
In the geological disposal concept of radioactive wastes, a kind of clay with sorption ability and low permeability, called bentonite, is envisaged as an engineered barrier system in the geological repository. Also, the cemetitious material is envisaged as the backfill material in the vaults and the structure material of the vaults. The groundwater in contact with the cementitious material will promote hyperalkaline conditions in the repository environment and these conditions will affect the performance of the bentonite. Therefore, it is necessary to investigate the interaction between the cementitious material and the bentonite for the evaluation of long term stability of the disposal system. In this study, for the identification and the investigation of the secondary minerals, the batch immersion experiments of the powder bentonite were carried out using synthetic cement leachates (pH=7, 12.5, 14) at 200 C. As the results, it was confirmed that Na as exchangeable cations in the bentonite can exchange relatively easily with Ca in the solution from the experiment results. And the ratio of cation exchange was estimated to be about 25% based on the amount of exchangeable cations Ca
C. As the results, it was confirmed that Na as exchangeable cations in the bentonite can exchange relatively easily with Ca in the solution from the experiment results. And the ratio of cation exchange was estimated to be about 25% based on the amount of exchangeable cations Ca between layers. Furthermore, it was concretely shown that the generation of analcime might be affected by the Na concentration from results of the solution analyses and a stability analysis of analcime using the chemical equilibrium model, in addition to the pH in the solution.
between layers. Furthermore, it was concretely shown that the generation of analcime might be affected by the Na concentration from results of the solution analyses and a stability analysis of analcime using the chemical equilibrium model, in addition to the pH in the solution.
JNC TN8400 2001-008, 36 Pages, 2001/03
Research on geologic disposal of high-level radioactive waste(HLW) has been underway in many countries. Bentonite exhibiting a low permeability, high swelling property and high sorption capacity for many radioelements is proposed as a buffer material in many countlies. Recently, cementitious materials are considered as candidate matelials for the geologic disposal of high-level radioactive waste. As the pH and the Ca, Na, K contents of hyperalkaline pore water from the cementitious materials are high, this hyperalkaline pore water would alter the buffer material. The main aim of this study is to investigate the effect of alkaline pore water into the bentonite. Used materials are montmorillonite, albite and quartz composing bentonite. These minerals mixed in a constant ratio (1:1wt%) made to react to distilled water and the alkali solutions (pH11-13). These studies have been conducted at temperatures of 50 - 150 C and run times of 10 - 200 day. XRD(X-ray powder diffraction) and SEM (Scanning Electron Microscopy) analyses were applied to studying the structure and quantitative data of each sample. From the result of this study, the main formed mineral of this experiment was analcime, which showed the tendency with a large amount of generation at a higher pH and temperature. Quantitative data of this study was conducted by X-ray powder diffraction method. THe order of the amount of the second analcime in each experiment is shown in the following. Montmorillonite and albite mixing test
C and run times of 10 - 200 day. XRD(X-ray powder diffraction) and SEM (Scanning Electron Microscopy) analyses were applied to studying the structure and quantitative data of each sample. From the result of this study, the main formed mineral of this experiment was analcime, which showed the tendency with a large amount of generation at a higher pH and temperature. Quantitative data of this study was conducted by X-ray powder diffraction method. THe order of the amount of the second analcime in each experiment is shown in the following. Montmorillonite and albite mixing test  Montmorillonite test
Montmorillonite test  Montmorillonite and quartz mixing test Activation energies (E
Montmorillonite and quartz mixing test Activation energies (E ) using the quantitative data of each test are shown in the following. (1)Montmorillonite test : 54.9kJ/mol (2)Montmorillonite and albite mixing test : 51.9kJ/mol (3)Montmorillonite and quartz mixing test : 59.6kJ/mol
) using the quantitative data of each test are shown in the following. (1)Montmorillonite test : 54.9kJ/mol (2)Montmorillonite and albite mixing test : 51.9kJ/mol (3)Montmorillonite and quartz mixing test : 59.6kJ/mol
Owada, Hitoshi*; Mihara, Morihiro; Iriya, Keishiro*; *
JNC TN8400 99-057, 43 Pages, 2000/03
Cementitious materials are considered as candidate materials for the geological disposal of high-level radioactive waste and TRU waste. As the pH and the Ca content of leachate from the cementitious materials are high, the host rock and the buffer-material would be degraded by the leachate in the long-term. Therefore, transport properties and parameters such as solubilities and distribution coefficients of radionuclides would be changed and affect the performance of the repository. In order to dissolve this "High pH plobrem", the use of a low alkalinity cement is considered for the disposal. In this study, we summarized the necessity of the low alkalinity cement, and developed the approach of the low alkalinization of cement. And, the following were carried out in this study : A leaching test of cement paste, a fluid test of the mortar and a installation test of the concrete to the trial structure. From the leaching test using the cement paste, we confirmed that we were able to obtain the low alkalinity cement (HFSC) by addition of pozzolanic materials such as silica-fume and flyash. From the result of the fluid test of the mortar, we chose the cement for the practicability evaluation. The practicability of low alkalinity concrete was evaluated by installation test to the trial structure.As a result of these examinations, we proved that the pH value of the leachate from the cementitious material was reduced by adding SF and FA to Portland cement. Simultaneously, SF and FA had to be added in order to obtain the good workability. In addition, workability and mechanical strength of the cement which SF and FA were added are almost equivalent to the ordinary Portland cement. The results shows that the HFSC has high practicability.
Sasamoto, Hiroshi; Yui, Mikazu; Arthur, R. C,*
JNC TN8400 99-033, 153 Pages, 1999/07
The results of hydrochemical investigations of groundwaters in the Kurihashi granodiorite at JNC's Kamaishi in-situ tests site indicate that these solutions are: (1)meteoric in origin, (2)chemically reducing (at depths greater than a few hundreds meters), (3)relatively young [residence times in the Kurihashi granodiorite generally less than about 40 years, but groundwaters older than several thousand years BP (before present) are also indicated by preliminary carbon-14 dating of samples obtained from the KH-1 borehole], (4)Ca-HCO type solutions near the surface, changing to Na-HCO
type solutions near the surface, changing to Na-HCO type groundwaters with increasing depth. The evolution of groundwater compositions in the Kurihashi granodiorite is modeled assuming local equilibrium for selected mineral-fluid reactions, taking into account the rainwater origin of these solutions. Results suggest it is possible to interpret approximately the "real" groundwater chemistry (i.e., pH, Eh, total dissolved concentrations of Si, Na, Ca, K, AI, carbonate and sulfate) in the Kurihashi granodiorite if the following assumptions are adopted: (1)CO
type groundwaters with increasing depth. The evolution of groundwater compositions in the Kurihashi granodiorite is modeled assuming local equilibrium for selected mineral-fluid reactions, taking into account the rainwater origin of these solutions. Results suggest it is possible to interpret approximately the "real" groundwater chemistry (i.e., pH, Eh, total dissolved concentrations of Si, Na, Ca, K, AI, carbonate and sulfate) in the Kurihashi granodiorite if the following assumptions are adopted: (1)CO concentration in the gas phase contacting pore solutions in the overlying soil zone = 10
concentration in the gas phase contacting pore solutions in the overlying soil zone = 10 bar, (2)minerals in the rock zone that control the solubility of respective elements in the groundwater include; chalcedony (Si), albite (Na), kaolinite (Al), calcite (Ca and carbonate), microcline (K) and pyrite (Eh and sulfate). Discussions with international experts suggest a systematic approach utilizing reaction-path models of irreversible water-rock interactions in open systems may be needed to more realistically model groundwater evolution at the Kamaishi test site. Detailed information characterizing certain site properties (e.g., fracture mineralogy) may be required to adequately constrain such models, however.
bar, (2)minerals in the rock zone that control the solubility of respective elements in the groundwater include; chalcedony (Si), albite (Na), kaolinite (Al), calcite (Ca and carbonate), microcline (K) and pyrite (Eh and sulfate). Discussions with international experts suggest a systematic approach utilizing reaction-path models of irreversible water-rock interactions in open systems may be needed to more realistically model groundwater evolution at the Kamaishi test site. Detailed information characterizing certain site properties (e.g., fracture mineralogy) may be required to adequately constrain such models, however.
*
PNC TJ1211 95-007, 117 Pages, 1995/02
1. Survey on leaching rate of pyrite, chlorite, epidote and siderite. (1) Pyrite Reaction rate (K) is depended on dissolve O concentration in Lin's literature. 1 - (1-x)
concentration in Lin's literature. 1 - (1-x) 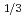 = k [O
= k [O ]
] 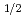 ・t (x: Mole number of dissolved FeS
・t (x: Mole number of dissolved FeS , [O
, [O ]: Dissolve O
]: Dissolve O concentration. T:Time) Reaction rate is changed by temperature. k = 2.2
concentration. T:Time) Reaction rate is changed by temperature. k = 2.2  10
10 exp(-9140/T) (2) chlorite Reaction rate is measured 2.7
exp(-9140/T) (2) chlorite Reaction rate is measured 2.7  6.7
6.7  10
10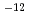 mol/m
mol/m /s(pH3
/s(pH3  4.5) by Swoboda-Colberg et al. Reaction rate is controlled by inner diffusion. (3) Epidote Reaction rate is measured 10
4.5) by Swoboda-Colberg et al. Reaction rate is controlled by inner diffusion. (3) Epidote Reaction rate is measured 10
 10
10 mol/cm
mol/cm /s in pH1
/s in pH1  11 by Rose. (4) Siderite Reaction rate is measured 9.93
11 by Rose. (4) Siderite Reaction rate is measured 9.93  10
10 mol/m
mol/m /s in O
/s in O -free solution by Greenberg et al. 2. Experimental study on leaching rate of minerals. (1) Measurement of leaching rate of minerals. Leaching rate of pyrite was measured in distilled waster under redox condition. Reaction rate (K) was estimated 10
-free solution by Greenberg et al. 2. Experimental study on leaching rate of minerals. (1) Measurement of leaching rate of minerals. Leaching rate of pyrite was measured in distilled waster under redox condition. Reaction rate (K) was estimated 10
 10
10 (cm
(cm ・mol
・mol ) 1/2・h
) 1/2・h by Lin's rate equation. At 56 days in the experiments. Alterated layer could not be found in SEM and EDX observation. (2) Measurement of leaching rate of chlorite Leaching rate of chlorite was measured in distilled waster under air condition. Disolution process of chloric was parabolic Stage, and reaction rate (K) was estimated order of 10
by Lin's rate equation. At 56 days in the experiments. Alterated layer could not be found in SEM and EDX observation. (2) Measurement of leaching rate of chlorite Leaching rate of chlorite was measured in distilled waster under air condition. Disolution process of chloric was parabolic Stage, and reaction rate (K) was estimated order of 10
 10
10 by Ross's rate equation.
by Ross's rate equation. 
 = kt [
= kt [  : disoleved mineral amount / intial mineral amount, t : time(sec) ] At 56 days in the experiments, alterated layer could not be found in SEM and EDX observation.
: disoleved mineral amount / intial mineral amount, t : time(sec) ] At 56 days in the experiments, alterated layer could not be found in SEM and EDX observation.